I-graphite, ifomula yamangqamuzana: C, isisindo samangqamuzana: 12.01, iwuhlobo lwe-elementi ye-carbon, i-athomu yekhabhoni ngayinye ixhunywe ngamanye ama-athomu ekhabhoni amathathu (ahlelwe ngamaheksagoni wezinyosi) ukuze akhe i-molecule ehlangene. Ngenxa yokuthi i-athomu ngayinye yekhabhoni ikhipha i-electron, lezo ezikwazi ukuhamba ngokukhululeka, ngakho i-graphite iyi-conductor.
I-graphite ingenye yamaminerali athambile kakhulu, futhi ukusetshenziswa kwayo kuhlanganisa ukwenza umthofu wepensela nezinto zokugcoba. Ikhabhoni i-elementi engeyona eyensimbi etholakala kumjikelezo wesibili weqembu le-IVA lethebula le-periodic. I-graphite yakheka emazingeni okushisa aphezulu.
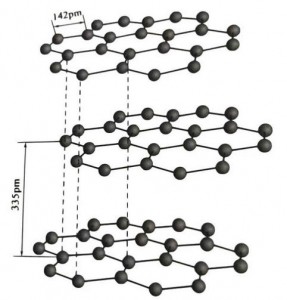
I-graphite iyi-crystalline mineral element ye-carbon elements, futhi i-crystalline lattice iyisakhiwo esinezingqimba ezine-hexagonal. Ibanga eliphakathi kwesendlalelo ngasinye semeshi ngu-3.35A, futhi ukukhala kwama-athomu ekhabhoni kusendlalelo semeshi efanayo ngu-1.42A. Iwuhlelo lwekristalu olunezinhlangothi ezine ezinezingqimba ezigcwele zokuqhekeka. Ingaphezulu le-cleavage liyizibopho zamangqamuzana, azikhangi kangako kuma-molecule, ngakho ukuntanta kwayo kwemvelo kuhle kakhulu.
Kumakristalu e-graphite, ama-athomu ekhabhoni akusendlalelo esifanayo akha isibopho esivumelanayo ne-sp2 hybridization, futhi i-athomu yekhabhoni ngayinye ixhunywe kwamanye ama-athomu amathathu kumabhondi amathathu aqinile. Ama-athomu ekhabhoni ayisithupha akha indandatho eqhubekayo eyisithupha endizeni efanayo, idlulela esakhiweni se-lamella, lapho ubude bebhondi yebhondi ye-CC bungu-142pm, okungaphakathi ncamashi kobude bebanga lebhondi lekristalu ye-athomu, ngakho-ke ngesendlalelo esifanayo. , iyikristalu ye-athomu. Ama-athomu ekhabhoni endizeni efanayo ane-p orbit eyodwa, edlulana. Ama-electron akhululekile, alingana nama-electron amahhala ezinsimbi, ngakho i-graphite ingakwazi ukuqhuba ukushisa nogesi, okuyisici samakristalu ensimbi. Kanjalo futhi kuhlukaniswa njengamakristalu ensimbi.
Ungqimba oluphakathi lwekristalu ye-graphite luhlukaniswe ngu-335pm, futhi ibanga likhulu. Ihlanganiswe ne-van der Waals force, okungukuthi, ungqimba luyingxenye yekristalu yamangqamuzana. Kodwa-ke, ngenxa yokuthi ukuboshwa kwama-athomu ekhabhoni kungqimba olufanayo lwendiza kunamandla kakhulu futhi kunzima kakhulu ukukubhubhisa, indawo yokuhlakazeka yegraphite nayo iphakeme kakhulu futhi izakhiwo zayo zamakhemikhali zizinzile.
Uma kubhekwa imodi yayo ekhethekile yokuhlanganisa, ayikwazi ukubhekwa njengekristalu eyodwa noma i-polycrystal, i-graphite manje ibhekwa njengekristalu exubile.
Isikhathi sokuthumela: Jul-31-2023